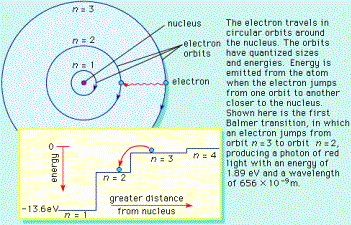
Energy Levels in Atoms
We can think
of an atom as consisting of a positive nucleus (protons and neutrons) surrounded by negative electrons. The electrons can be
thought of as “orbiting” the nucleus, but
are only allowed in certain orbits (or energy levels).
A photon with exactly the right energy can excite the
electron from one level to another. The
electron will drop back to the “ground state”, and emit photons with specific energies as it does so.
“Chemistry” is
caused by the fact that no 2 identical electrons can be in the same orbital at the same time.